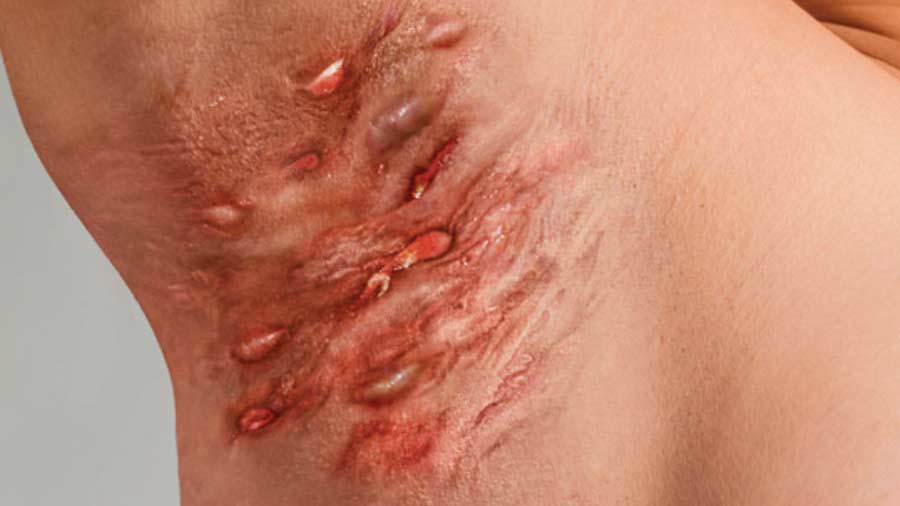
New Trial Added: Hidradenitis Suppurativa
We are currently looking for participants in a Hidradenitis Suppurativa clinical trial being conducted at our Johnston, RI location.
Do you or someone you know suffer from moderate to severe Hidradenitis Suppurativa, also known as HS? Dr. Ellen Frankel and Clinical Partners are looking for participants ages 18 and older with a diagnosis of Hidradenitis Suppurativa to evaluate an investigational medication. Medical Insurance is not required and there is no cost to participate. Patients that qualify and enroll into the study may be compensated for time and travel.